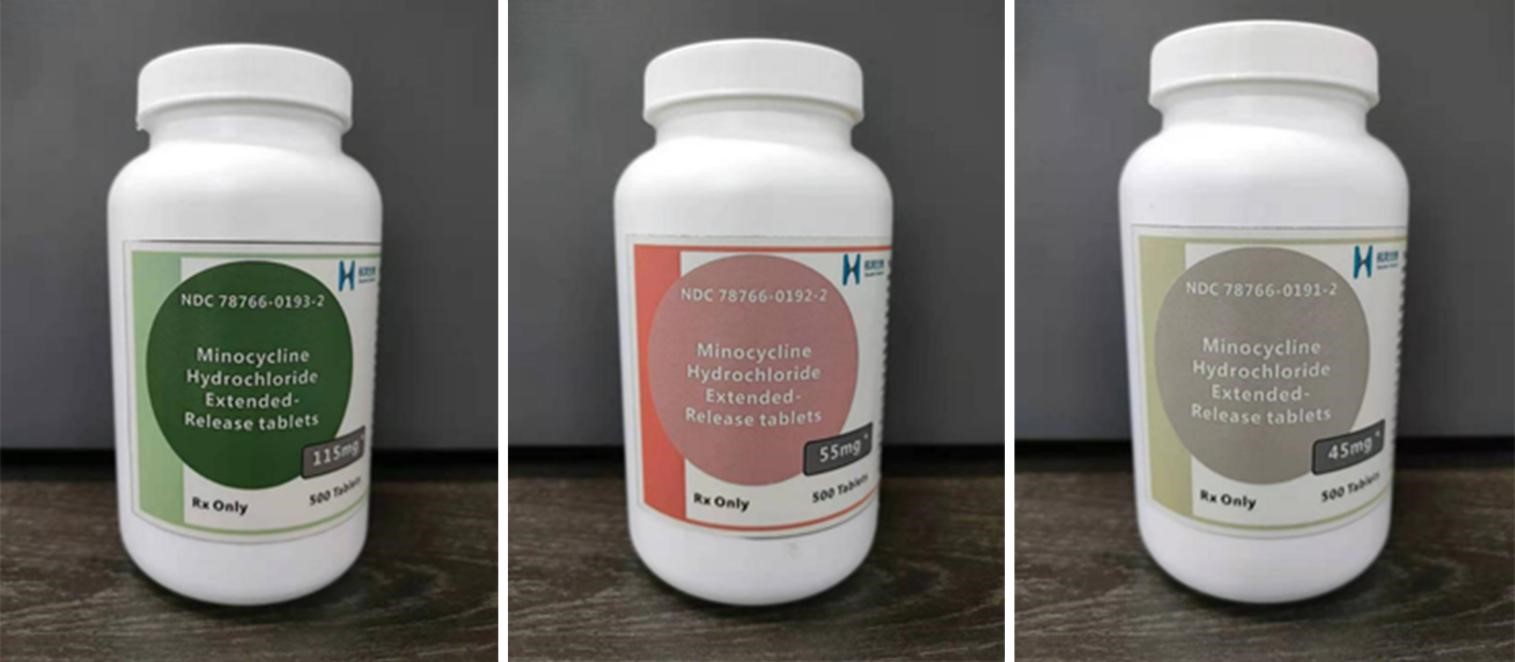
Minocycline Hydrochloride Extended-Release Tablet
Product Name:Minocycline Hydrochloride Extended-Release Tablet
Dosage form:Tablets/oral
Specifications:45mg、55mg、115mg
Product declaration category:ANDA
Indication:It is mainly applicable to infections caused by staphylococcus, streptococcus, pneumococcus, Neisseria gonorrhoeae, dysentery bacillus, Escherichia coli, Klebsiella, Proteus, Pseudomonas aeruginosa, Treponema pallidum, chlamydia and other pathogens sensitive to this product
Clinical use:① Treat acne. ② Treat non gonococcal urethritis. ③ Therapeutic use in stomatological diseases. ④ Treat prostatitis. ⑤ Treat male infertility.
Dosage form:Tablets/oral
Specifications:45mg、55mg、115mg
Product declaration category:ANDA
Indication:It is mainly applicable to infections caused by staphylococcus, streptococcus, pneumococcus, Neisseria gonorrhoeae, dysentery bacillus, Escherichia coli, Klebsiella, Proteus, Pseudomonas aeruginosa, Treponema pallidum, chlamydia and other pathogens sensitive to this product
Clinical use:① Treat acne. ② Treat non gonococcal urethritis. ③ Therapeutic use in stomatological diseases. ④ Treat prostatitis. ⑤ Treat male infertility.
Project Schedule:Minocycline Hydrochloride Extended-Release tablets complete bioequivalence clinical experiment(BE)
The bioequivalence test of minocycline hydrochloride Extended-Release tablets was completed in the Canadian clinical laboratory company Syneos Health。The experimental data showed that the bioequivalence consistency of minocycline hydrochloride Extended -release tablets (115mg) developed by MHDC and SOLODYN (115mg) produced by the original American company MEDICIS PHARMACEUTICAL CORP met FDA requirements.
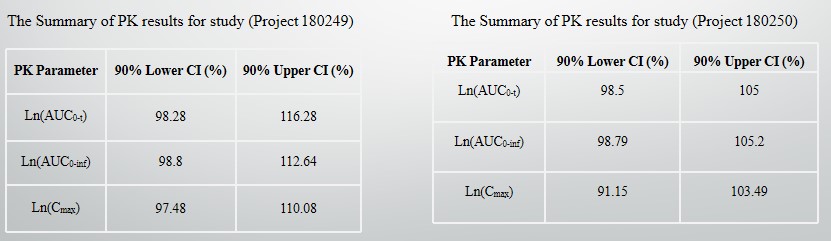 (180249 refers to pre meal data and 180250 refers to post meal data)
(180249 refers to pre meal data and 180250 refers to post meal data)
 (180249 refers to pre meal data and 180250 refers to post meal data)
(180249 refers to pre meal data and 180250 refers to post meal data)
FDA submission: Minocycline Hydrochloride Extended-release Tablets have been successfully submitted to the US ANDA, and have been initially confirmed by the FDA review, and will be marketed in the US in 2023.