GMP Industrialization Construction

Industrial Manufacturing-Zhejiang Bayside Biotech Co., Ltd
- 30min by car from MHDC
- Completion & delivery by the end of 2021
- 7500 square meter standard factory
- Substantive Design completed
- Planned 4 cGMP production line:
1、Complex LAR injectable
2、High-level solid dosage form
3、Oral solution/injectable
4、Other
The construction of the company's first GMP production line of Microsphere LAR has been basically completed, which can not only ensure the industrialization of the company's microsphere products, with a production capacity of 25000 vial/batch,but also undertake the cooperation projects of other pharmaceutical enterprises.

The design of solid extended-release drugs is completed, and it will be completed by August 2022 with a production capacity of 8 million pieces/year.
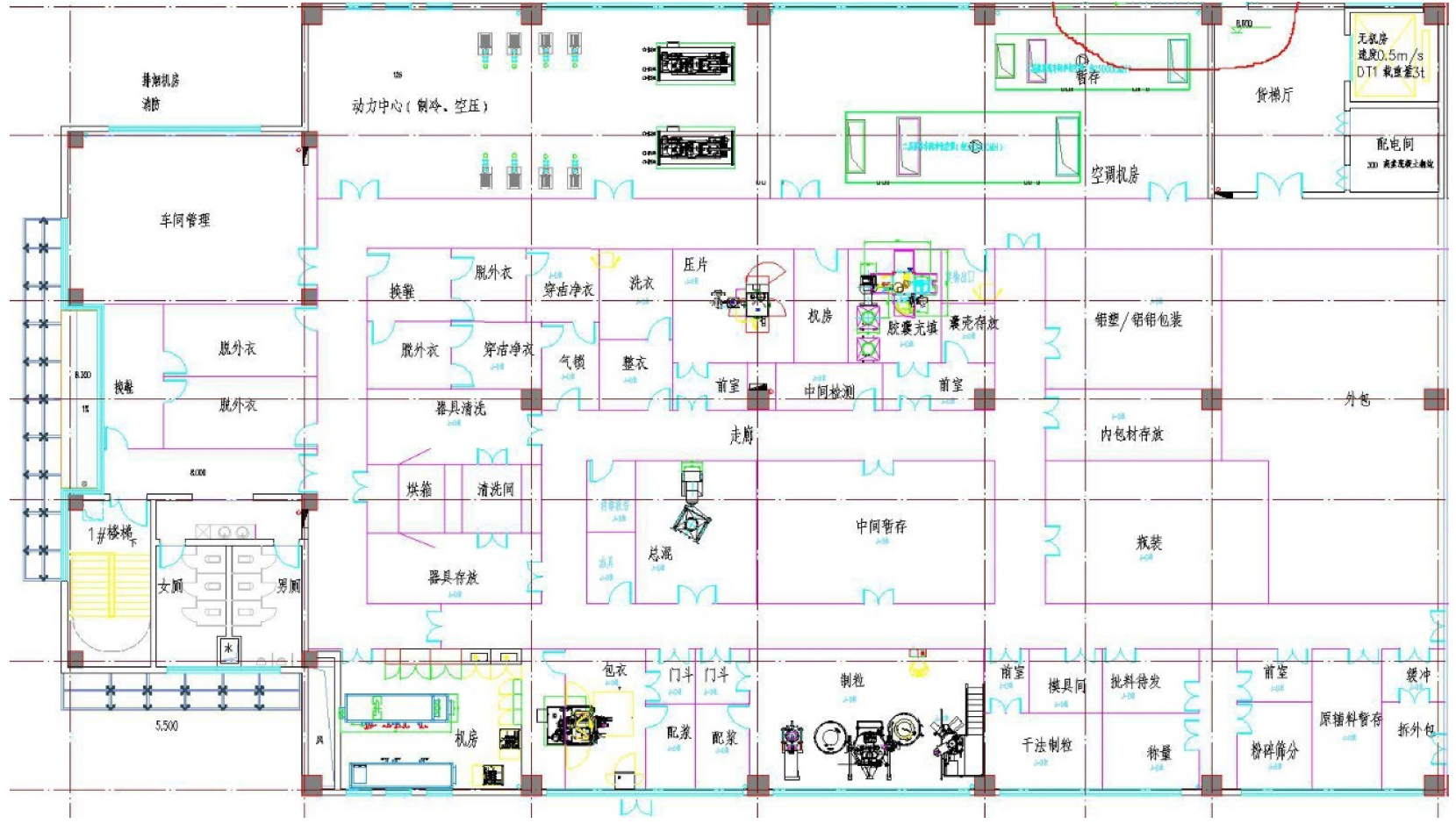
complete GMP scale-up

